The atomic absorption spectrophotometer: Precision in Elemental Analysis
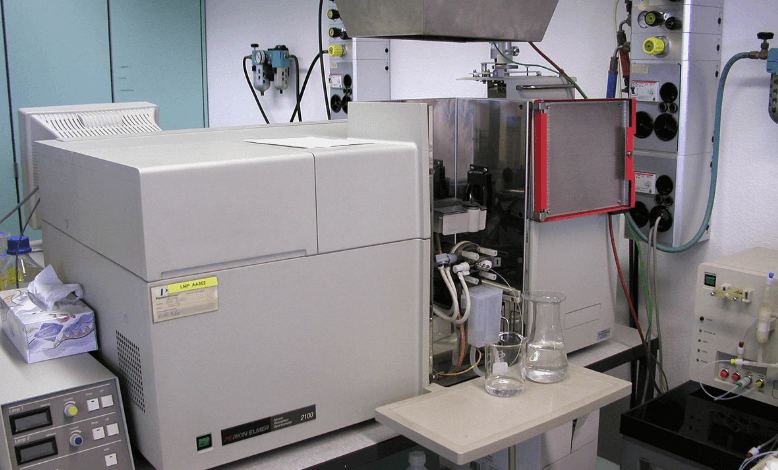
In the world of modern analytical chemistry, the ability to detect and quantify specific elements within a sample is vital across various industries. From environmental monitoring to pharmaceutical development, the demand for reliable, accurate, and cost-effective elemental analysis tools is higher than ever. One instrument that has remained a workhorse in this domain for decades is the atomic absorption spectrophotometer.
Known for its precision, ease of use, and affordability, the atomic absorption spectrophotometer continues to serve as an indispensable tool in labs around the globe. This article explores the fundamentals of this instrument, how it works, its key benefits, applications, and how it compares to other spectroscopic tools.
What is an Atomic Absorption Spectrophotometer?
An atomic absorption spectrophotometer is an analytical instrument used to determine the concentration of specific metal elements in liquid samples. It operates on the principle that atoms absorb light at characteristic wavelengths. When a sample is atomized and exposed to a specific wavelength of light, the atoms of the element of interest absorb some of that light. The amount of absorbed light is proportional to the element’s concentration in the sample.
Because of its accuracy and affordability, AAS has long been the go-to technology for trace metal analysis, especially in labs that require the quantification of a few elements at a time.
How Does an Atomic Absorption Spectrophotometer Work?
The operation of an atomic absorption spectrophotometer involves several key components and steps:
- Sample Preparation: The analyte (sample) is usually digested into a liquid solution containing the target metal ions.
- Atomization: The sample is introduced into a flame or graphite furnace, which converts the metal ions into free atoms.
- Light Source: A hollow cathode lamp specific to the target element emits a narrow beam of light at that element’s characteristic wavelength.
- Absorption of Light: The free atoms in the flame or furnace absorb light from the lamp.
- Detection: A photodetector measures the reduction in light intensity, which correlates with the element’s concentration.
- Output: The data is processed by the software and presented as numerical results and calibration curves.
See also: How AI Face Swap Technology Is Shaping User-Generated Content
Key Components of an Atomic Absorption Spectrophotometer
To better understand the instrument’s function, let’s examine its primary components:
- Hollow Cathode Lamp (HCL): Emits element-specific light; one lamp per element is generally required.
- Burner or Graphite Furnace: The atomization chamber; flame is used for general analysis, while graphite is used for ultra-trace detection.
- Monochromator: Filters out unwanted wavelengths, allowing only the desired spectral line to pass through.
- Detector: Measures the intensity of light reaching it after passing through the atomized sample.
- Data System: Converts raw detector signals into readable concentrations via calibration curves.
Advantages of Using an Atomic Absorption Spectrophotometer
The atomic absorption spectrophotometer remains a favorite in many labs for its many advantages:
✅ High Accuracy for Select Elements
It provides excellent accuracy when quantifying specific metals such as lead, zinc, copper, magnesium, and calcium.
✅ Cost-Effective
Compared to more complex spectrometric tools like ICP-MS, the atomic absorption spectrophotometer is affordable and requires minimal maintenance.
✅ Simplicity of Operation
Many AAS units are easy to use, making them accessible for labs without highly specialized technical staff.
✅ Low Detection Limits
Especially with graphite furnace atomization, AAS can detect trace metals in the parts-per-billion (ppb) range.
✅ Small Sample Requirements
A small amount of liquid sample is sufficient for accurate analysis.
Common Applications of the Atomic Absorption Spectrophotometer
The atomic absorption spectrophotometer is versatile and used in a wide variety of industries, including:
🌍 Environmental Testing
- Monitoring heavy metals in drinking water, wastewater, and soil.
- Compliance with environmental safety standards (EPA, ISO, WHO).
💊 Pharmaceutical Quality Control
- Determining elemental impurities in raw materials and final drug formulations.
- Ensuring regulatory compliance under ICH Q3D guidelines.
🧪 Food and Beverage Industry
- Testing for toxic metals like arsenic and lead in food and drinks.
- Nutrient analysis in dairy products and fortified beverages.
🏭 Mining and Metallurgy
- Analysis of ores, minerals, and alloy compositions.
- Quality control in metal processing and manufacturing.
🌱 Agriculture and Fertilizer Testing
- Soil nutrient profiling (e.g., potassium, calcium, magnesium).
- Trace metal detection in fertilizers and crop samples.
Types of Atomic Absorption Spectrophotometers
AAS instruments are available in two main configurations:
1. Flame Atomic Absorption Spectrophotometer (FAAS)
- Uses a flame (typically air-acetylene) for atomization.
- Suitable for ppm-level analysis.
- Ideal for general-purpose labs.
2. Graphite Furnace Atomic Absorption Spectrophotometer (GFAAS)
- Uses an electrically heated graphite tube.
- Offers much lower detection limits (ppb range).
- Best for trace analysis and small sample volumes.
Some advanced instruments are hybrid systems, combining flame and furnace capabilities in one platform for greater flexibility.
AAS vs Other Spectrometric Techniques
Here’s how the atomic absorption spectrophotometer compares with other common elemental analysis tools:
| Technique | Strengths | Limitations |
| AAS | Affordable, accurate, easy to use | One element at a time |
| ICP-OES | Multi-element, fast, moderate sensitivity | More expensive and complex |
| ICP-MS | Ultra-trace analysis, multi-element | High cost, high maintenance |
| XRF | Non-destructive, fast | Lower sensitivity for light elements |
If you’re focusing on routine analysis of a few metals at a time, AAS offers the most value for investment.
Tips for Maximizing AAS Performance
To get the best results from your atomic absorption spectrophotometer, follow these best practices:
- Use certified reference standards for calibration.
- Regularly clean the burner head to prevent signal interference.
- Perform daily background correction for accurate readings.
- Use matrix modifiers in graphite furnace methods to reduce interference.
- Check lamp alignment and intensity frequently for consistent output.
Choosing the Right AAS Instrument for Your Lab
Before investing in an atomic absorption spectrophotometer, consider the following:
- Elements to Analyze: Choose lamps and configurations based on your target elements.
- Detection Limits Needed: If you require trace-level detection, go for graphite furnace models.
- Sample Throughput: High-volume labs may benefit from autosamplers and faster processing speeds.
- Software Features: Look for user-friendly interfaces, real-time analytics, and data export functions.
- Budget and Support: Balance price with warranty, training, and after-sales service.
Final Thoughts
The atomic absorption spectrophotometer remains a reliable, accessible, and cost-effective tool for elemental analysis. Despite the rise of newer technologies like ICP-MS and ICP-OES, AAS continues to thrive, especially in labs focused on single-element or low-throughput analysis. Its accuracy, user-friendliness, and adaptability make it a foundational instrument in scientific research, quality control, and regulatory compliance.
If your lab is seeking precise metal quantification without the high complexity or cost of advanced plasma-based techniques, the atomic absorption spectrophotometer is a smart, proven choice.




